Products
FIB-SEM
Nanomanipulators
OmniProbeOmniProbe CryoSoftware
AZtec3DAZtecFeatureAZtec LayerProbeTEM
Hardware
EDSUltim MaxXploreImaging
TEM CamerasSoftware
AZtecTEM

The Pharmaceutical and Biomedical industries require EDS systems to show that their electronic records are trustworthy, reliable, and equivalent to paper records. AZtecPharma is designed to meet the growing requirements of the Pharmaceutical and Biomedical industries with:
Individual user login
Digital signatures
Data Audit Trail with viewer
Data tree deletion forbidden
AZtecPharma is a dedicated version of the market-leading AZtecLive software platform with increased data security and integrity. It enables EDS analysis to be incorporated into laboratory Standard Operating Procedures to support good practice quality guidelines like ‘GxP’ and regulations such as 21 CFR part 11 and Annex 11
Secure
Accurate
Traceable
Individual User Login
Digital Signature
Audit Trail and Inspector
Live Chemical Imaging
SOPs and Profiles
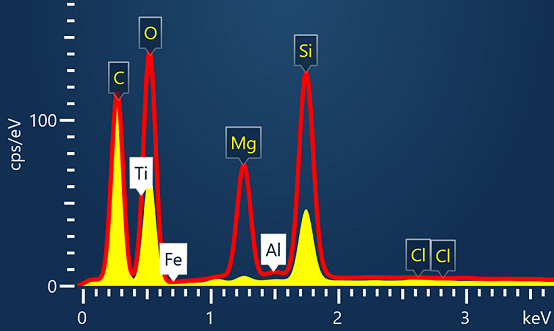
Main applications carried out in Pharmaceutical production facilities are Quality Control and Failure Analysis
Quality Control is often a simple check on the structure and chemical composition of the product. Using AZtec’s live spectrum compare is an ideal way of comparing a sample's composition with control. Any sample that deviates from the control will be submitted for more detailed Failure Analysis.
• Material from 3rd party supplier may contain contamination from numerous sources (Metals, plastics, glass, ceramics, fibres)
• Production process
• Cross-contamination from other products using same facility
• Contamination from equipment (metals, plastics, paint, oils etc…)
• Human contamination
• Contaminated blister packs, tubes, jars and boxes
• Defective Actuators and syringes
Foreign body identification in products is the most common application. Whether it’s from the raw materials or the production process; Tracing the contamination back to source is key to preventing future occurrences. This contamination can cause the product to fail or in the worst case, cause harm to the consumer. In the example below, an initial investigation was carried out using AZtecLive Chemical Imaging to get a quick overview of the tablet structure and composition. Then a more detailed analysis was carried out using spectrum compare and then X-Ray mapping.
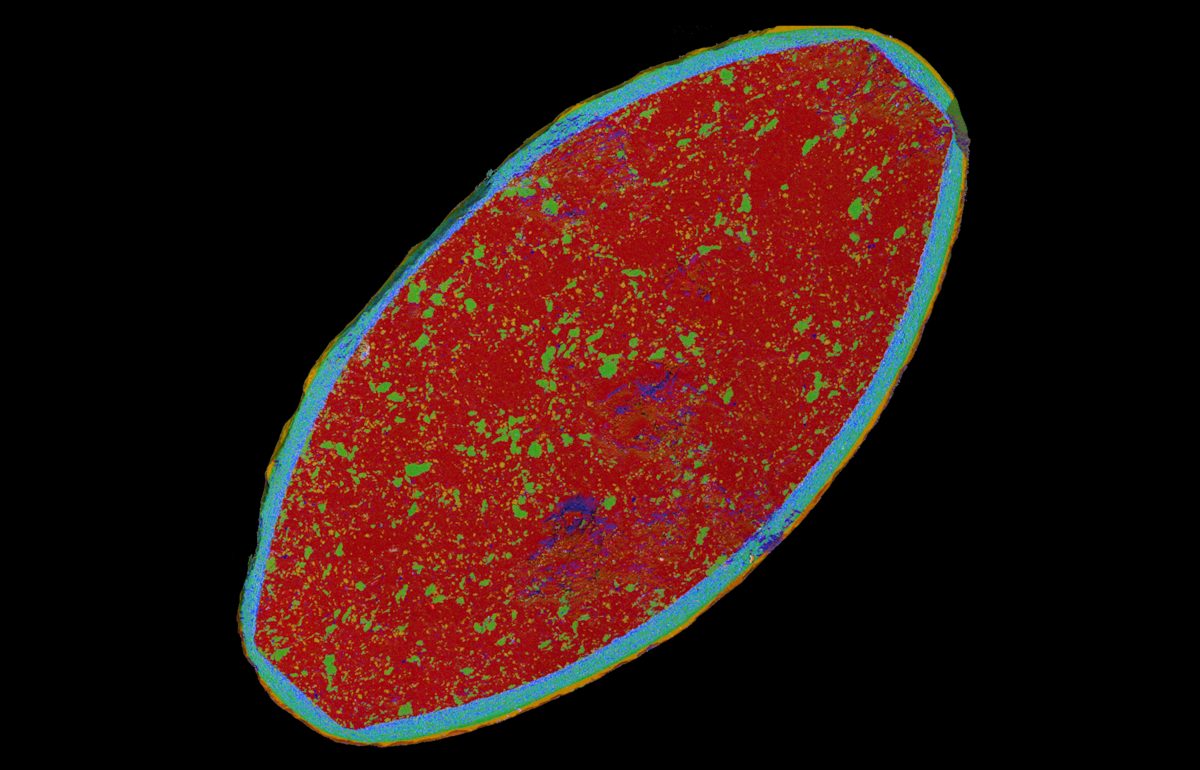
Packaging can be a source of contamination or be defective in a way that it allows external contamination of the product it encloses
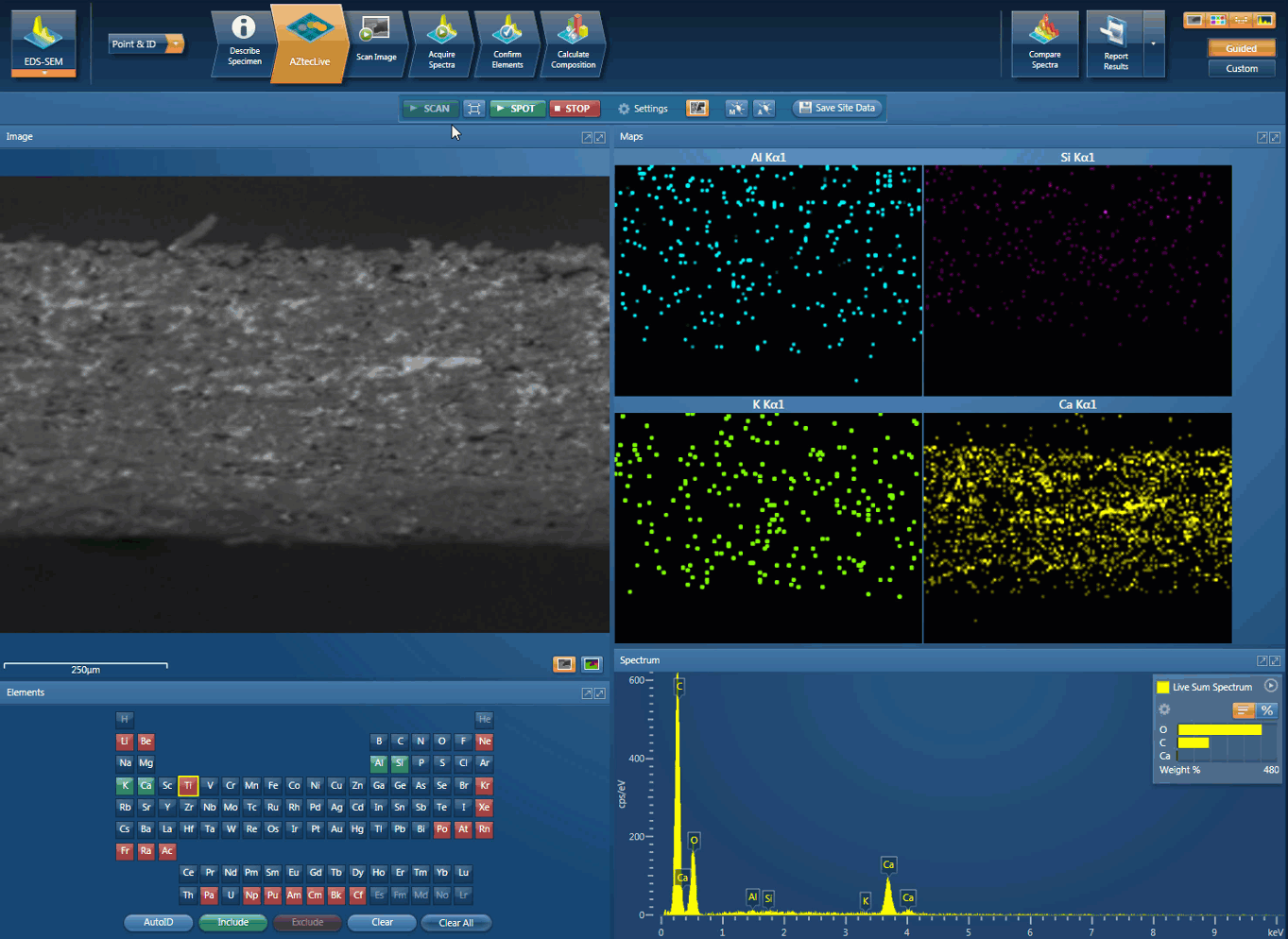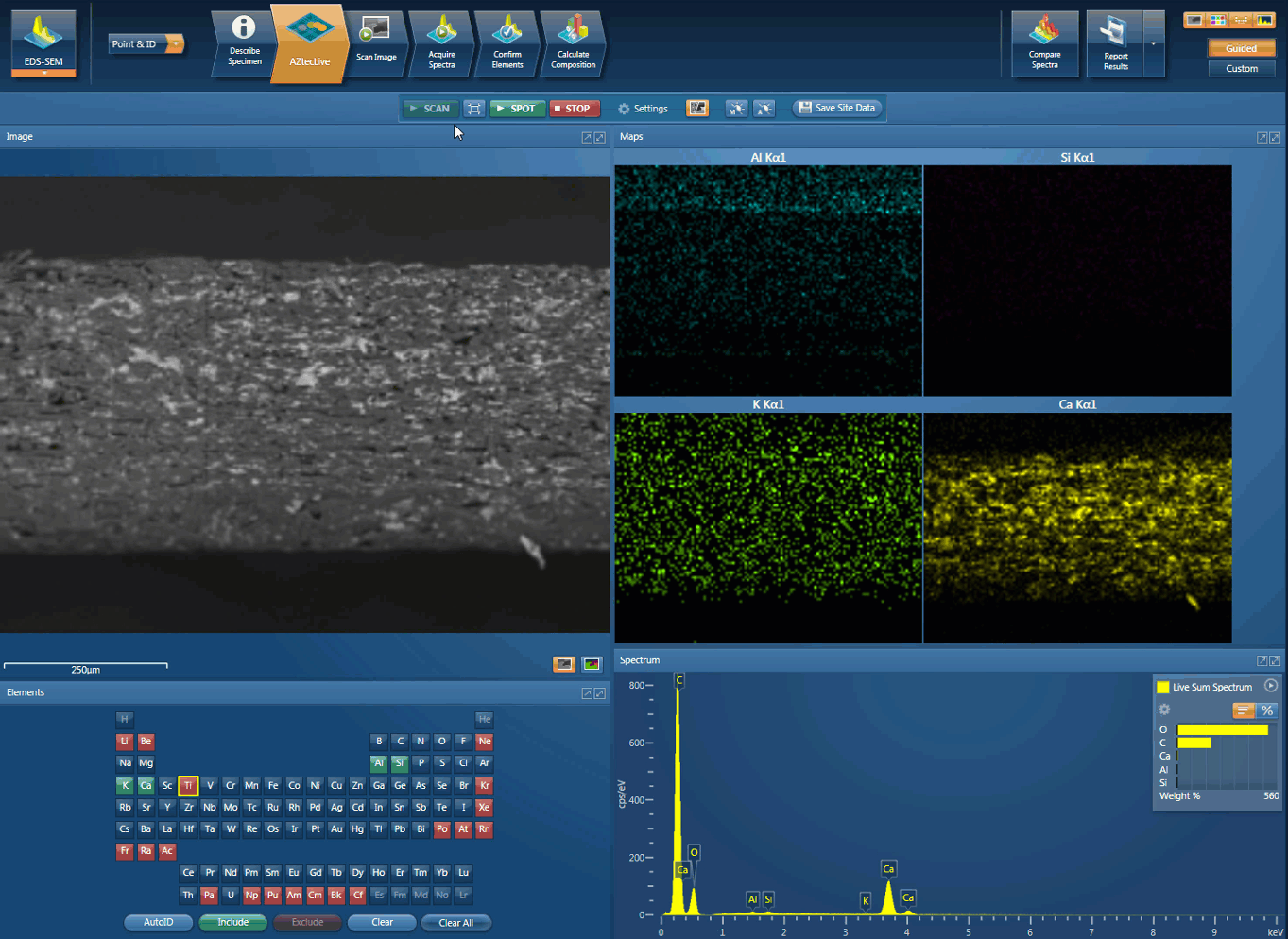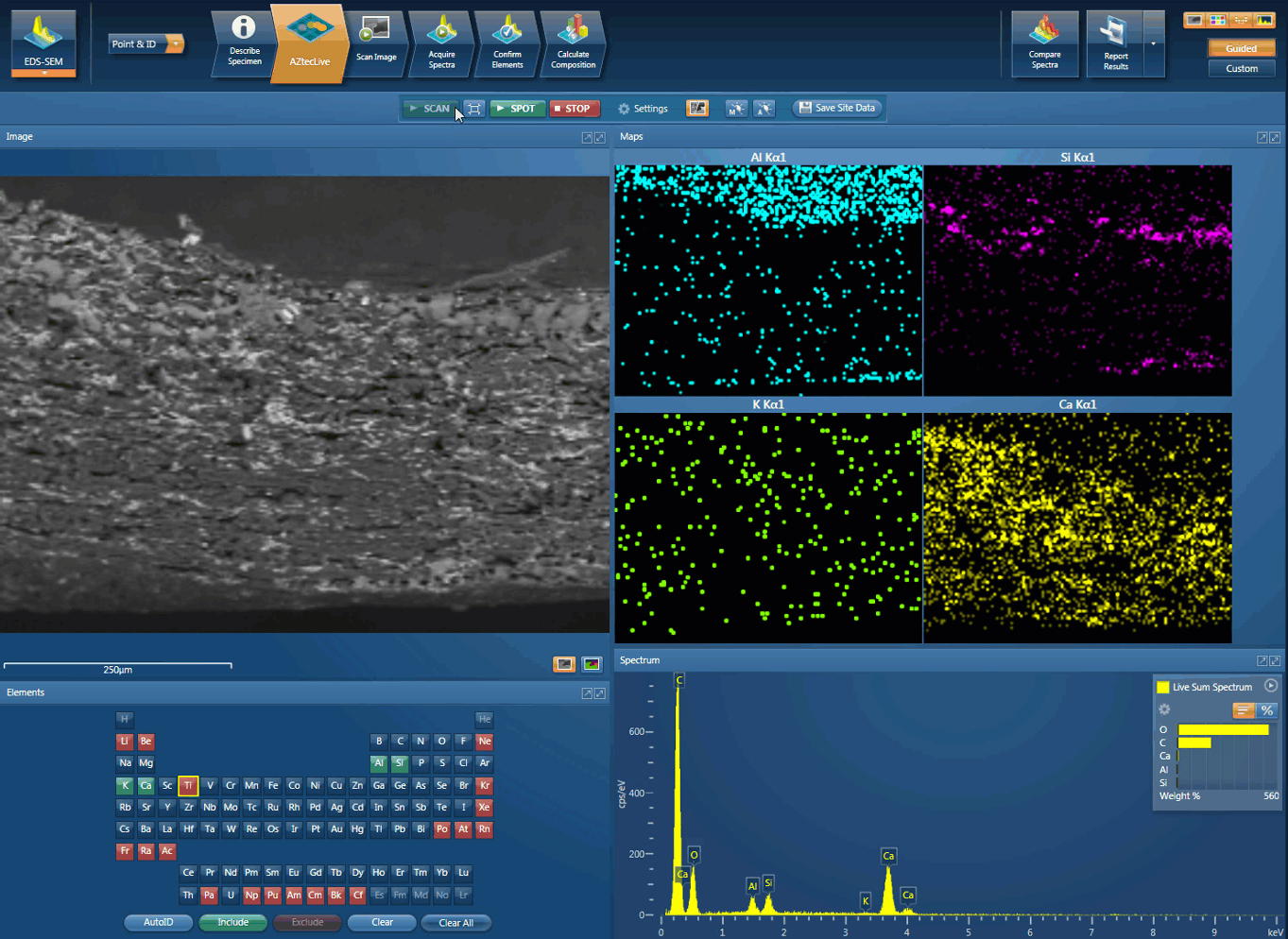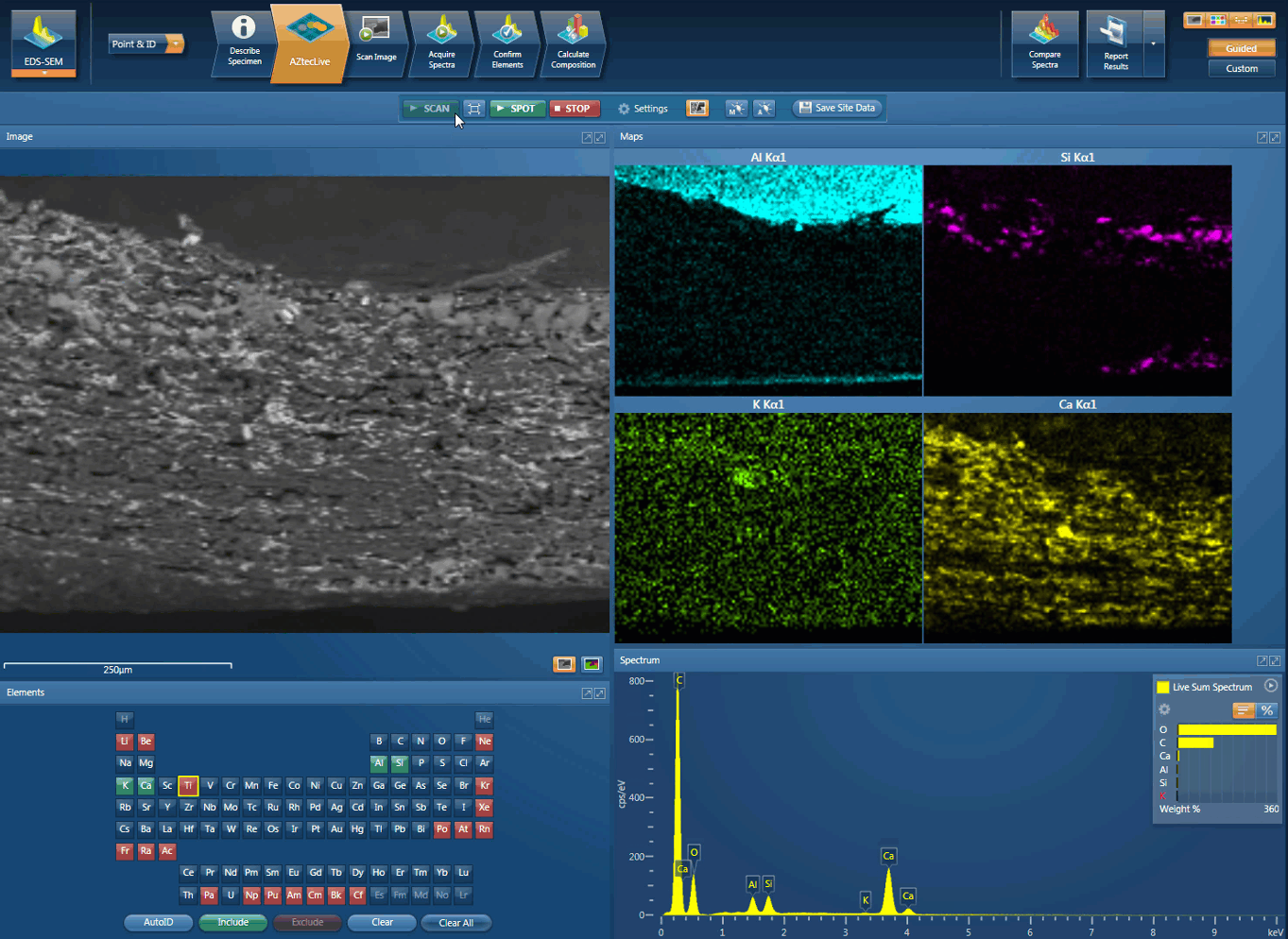
Movie showing an initial investigation can be carried out using AZtecLive Chemical Imaging
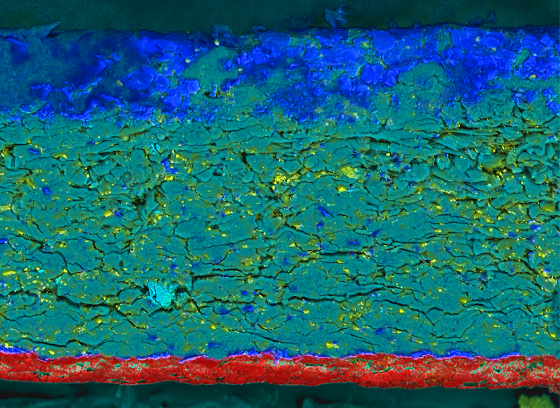
Layered image showing the chemical distribution over the cross section of cellulose packaging
The drug delivery mechanism can also be a potential source of contamination, for example:
· Failure of Asthma Cannister causing contamination fragments to contaminate the drug actuations
· Failure of vials and syringes resulting in glass contamination
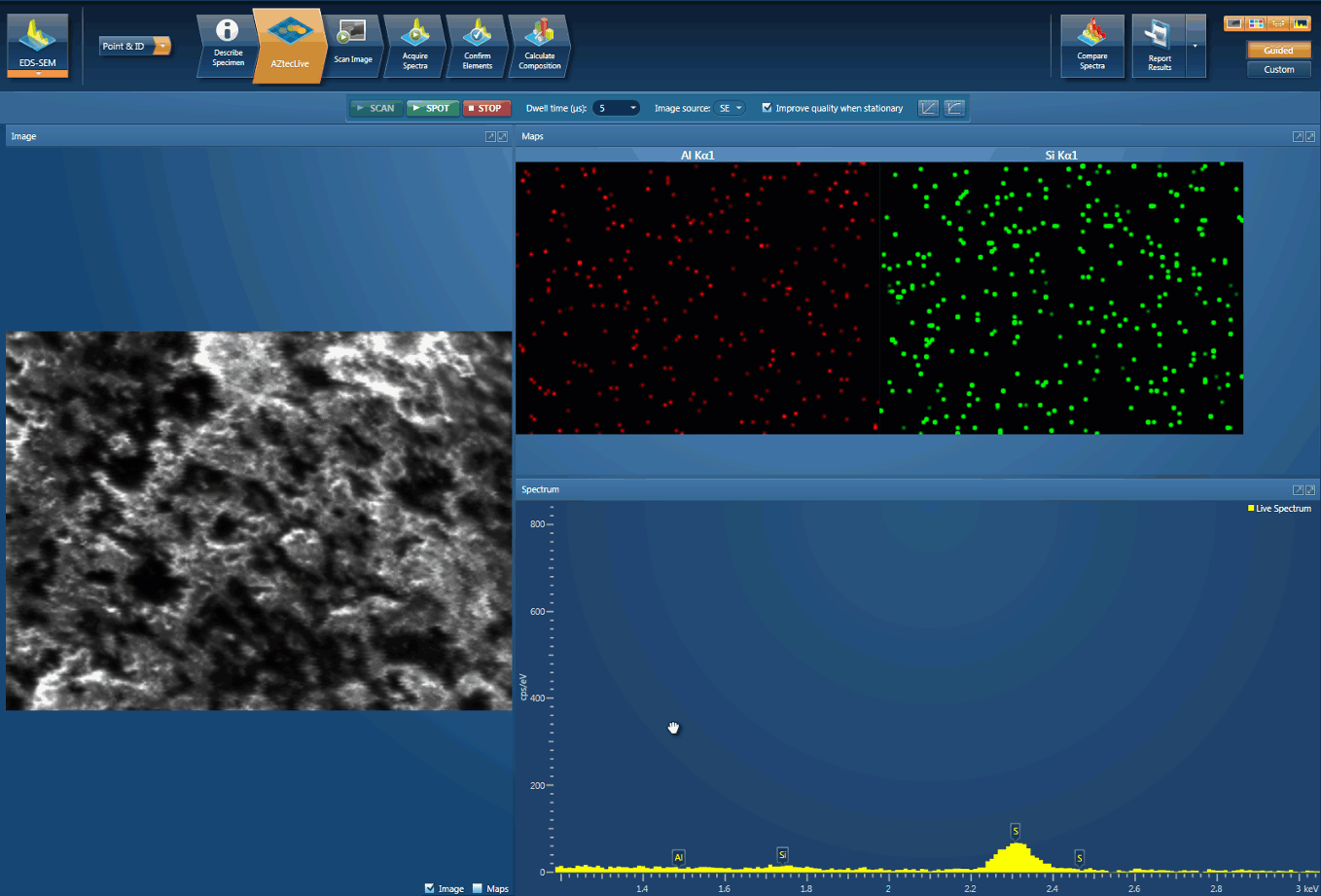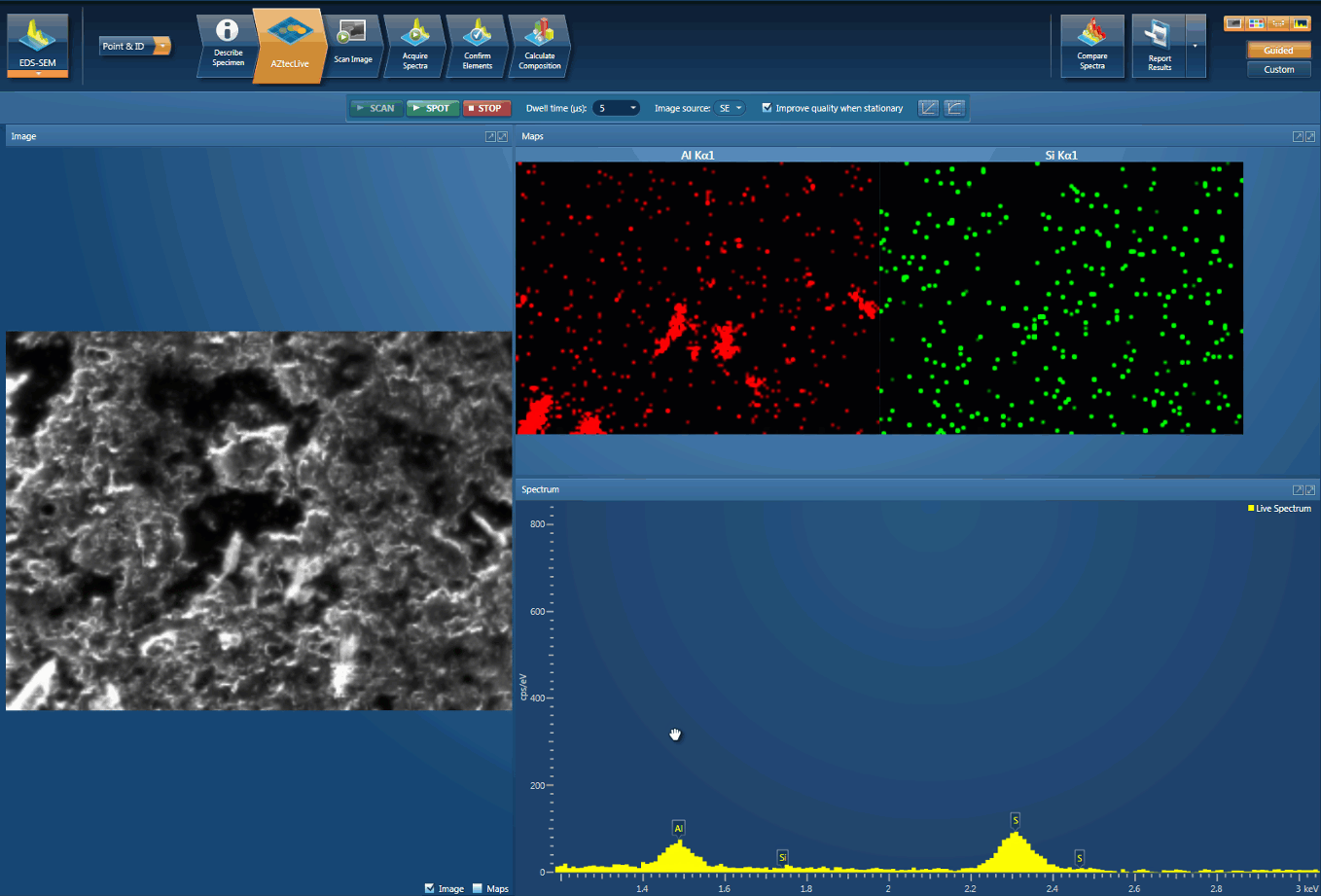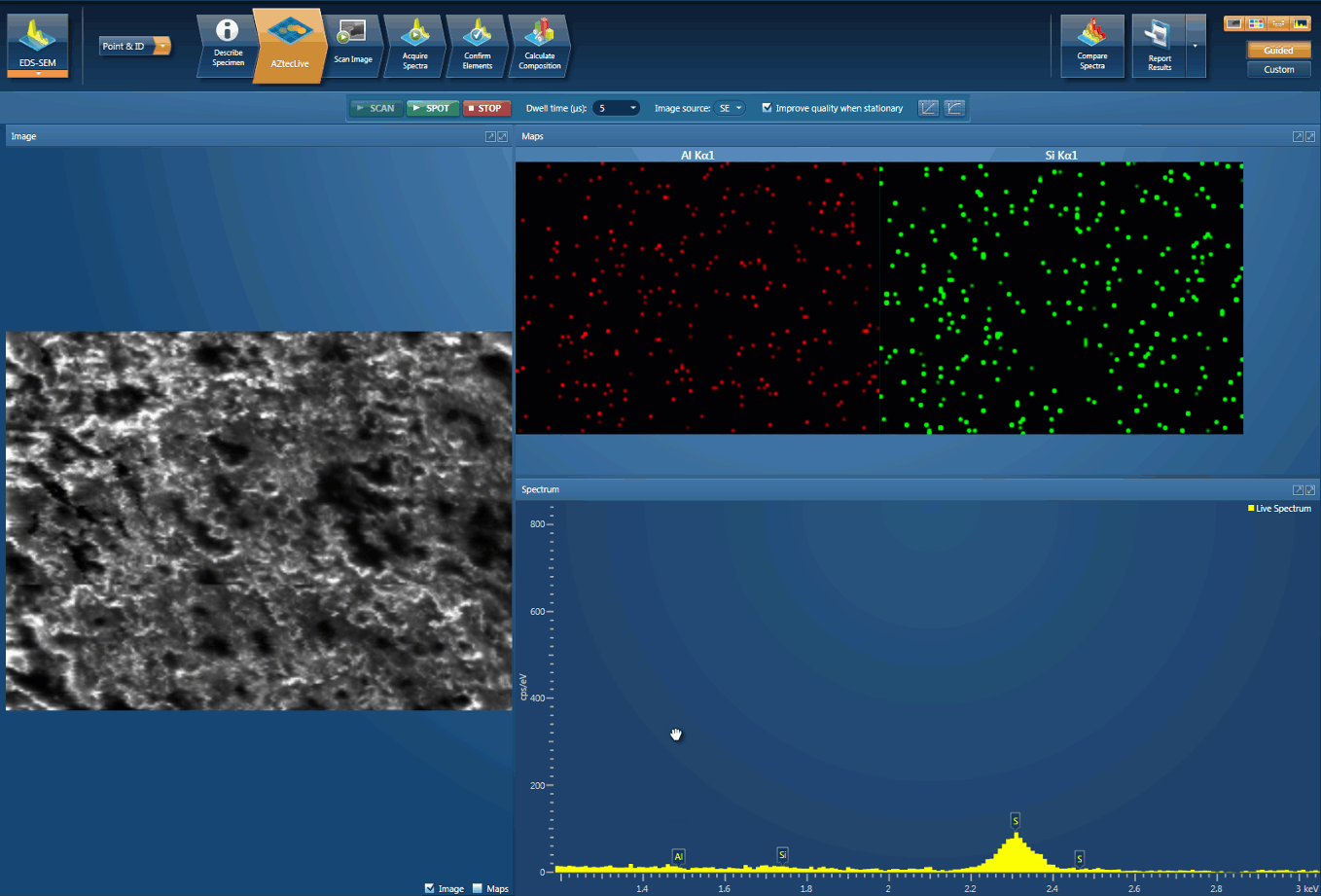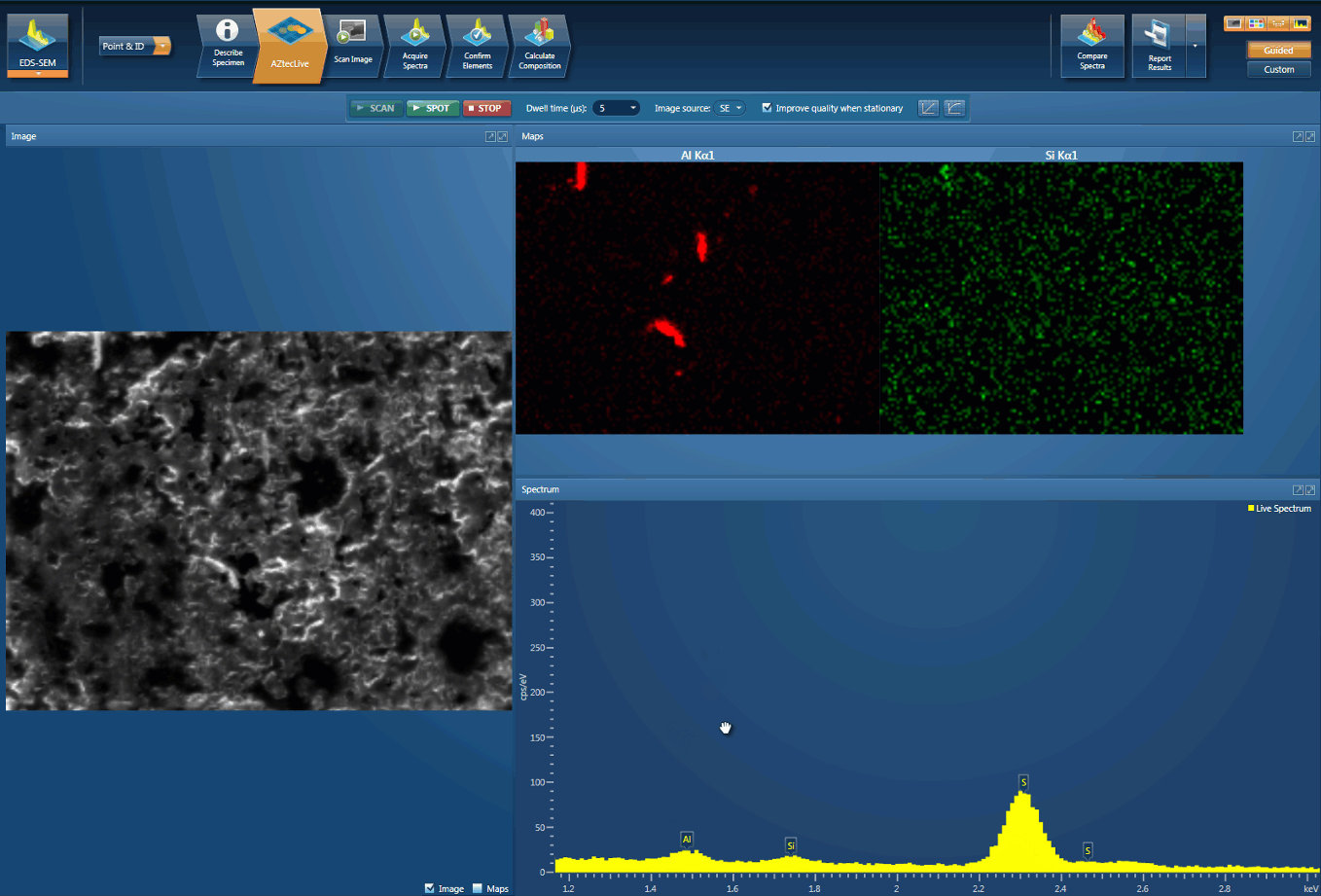
Detection of Al contamination particles from a defective inhaler

Actuation from an Asthma inhaler