Cryo electron microscopy (EM) is an area that has shown significant and sustained growth in recent years. A large proportion of this due to its suitability for imaging of frozen and hydrated biological materials, avoiding the artefacts common to room temperature sample preparation. However, cryo is not limited to biological applications: more materials scientists are using the technique to look at beam sensitive materials (such as polymers and III-V semiconductors) and vacuum incompatible materials (solid-liquid interfaces, like hydro gels and batteries).
A key feature of Cryo EM samples is that they are vitrified, meaning that the water present in the sample solidifies without the formation of ice crystals (i.e. it maintains its room temperature structure frozen). This is very important for biological samples, which are typically over 80% water, where the formation of ice crystals causes damage and artefacts. Vitrification is achieved by either plunge freezing (dropping a small sample in ethane or propane cooled by liquid nitrogen), slam freezing (bringing the sample into rapid contact with a liquid nitrogen cooled metal block) or high pressure freezing (HPF, the simultaneous application of high pressure and liquid nitrogen). Imaging the ultrastructure of frozen and hydrated samples at high resolutions requires maintaining a cold environment within the microscope and cryo TEM utilises specialised sample holders and imaging protocols to avoid warming the sample up.
TEM sample preparation at room temperature for all types of specimen in biological and materials sciences is often challenging. Cryo samples add an additional level of complexity. Cryo samples cannot be handled directly, must be kept at a temperature below -80°C, must not be exposed to humidity in the air and are extremely small and fragile. The samples must also be thin enough for the transmission of an electron beam. There are two established methods of producing thin cryo samples for TEM, cryo-ultramicrotomy (a microtome using a diamond knife to produce thin slices at cryo temperatures) and . Cryo-ultramicrotomy is an extremely specialised method that has a number of challenges, can introduce sample damage and has a limited ability to select specific regions within a sample. On grid cryo-FIB milling requires that the feature of interest is positioned in the central region of a TEM grid before freezing, which is not trivial due to sample size and requirements on handling. It also needs the full thickness of the frozen sample to be milled, which limits how the sample can be frozen and the types of sample that can be used, as HPF samples are too thick to mill and other freezing techniques are restricted to samples no larger than a single cell.
A common approach to site specific TEM lamella preparation of non-cryo samples is the in-situ FIB lift-out, detailed in a previous blog. This method uses a FIB to mill the sample and an OmniProbe to lift out the milled sample and transfer to a liftout grid. Once attached to the grid the sample is thinned. Sample attachment to the OmniProbe and grid occurs using beam assisted deposition. As the tip is at room temperature this method is not suitable for cryo applications and even with a cooled tip the conventional attachment methods will not work as the cryo conditions inside the microscope results in uncontrolled deposition.
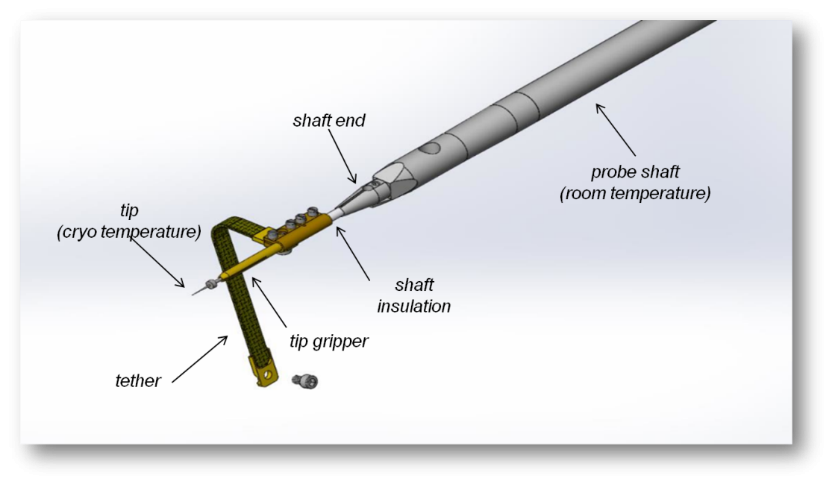
Early design of the cryo shaft used to perform cryogenic FIB liftout
However, the lift out process can be adapted by modifications to the OmniProbe nanomanipulator to work on cryogenic specimens, both biological and non-biological. Modifications to the OmniProbe enable cooling of the probe tip without additional hardware (except a cryo-stage in the microscope). The probe tip temperature should be cooler than the sample, but warmer than the anti-contaminator device (ACD) i.e. ACD < Probe < Sample. The ACD is designed such that any contamination in the chamber condenses on the ACD, not on other parts at cryogenic temperatures, and must be the coolest part of the system. The Omniprobe achieves this balance by using passive cooling of the probe via the ACD. The ACD’s are typically at least 20oC cooler than the sample, and the efficiency of the passive connections used mean that the probe is typically around 10oC warmer than the ACD.
The final challenge attaching the cooled probe to the sample. The traditional method of beam assisted deposition does not work as the gas injected into the chamber condenses on the sample as a thick layer, which fills the milled trenches. We have developed a method which uses a low vapour pressure gas, which when injected deposits on the sample and probe as, allowing attachment of the probe to the sample without contamination or damage of the liftout sample. The gas used is a standard GIS chemistry intended to be used to enhance the ion beam milling rates of carbon, not for deposition. Using this method means the same workflow can be used for both room temperature and cryo liftout, with only slight changes.
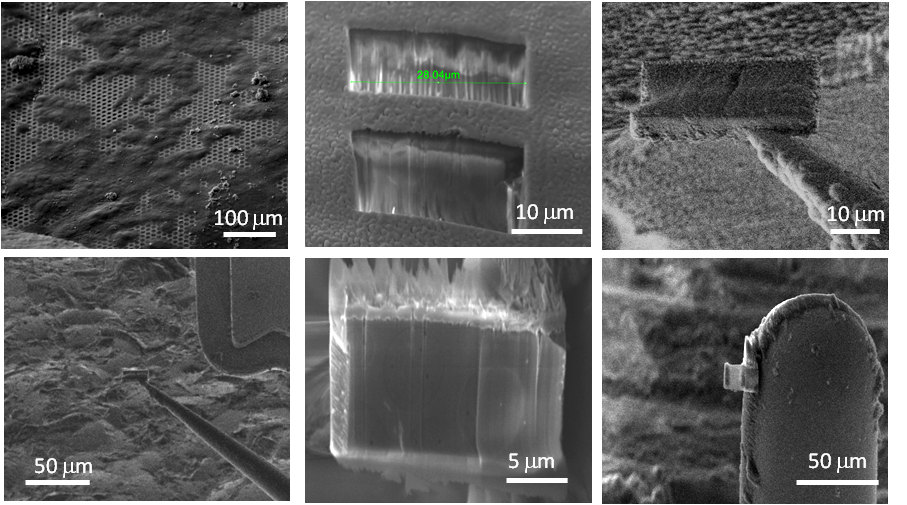
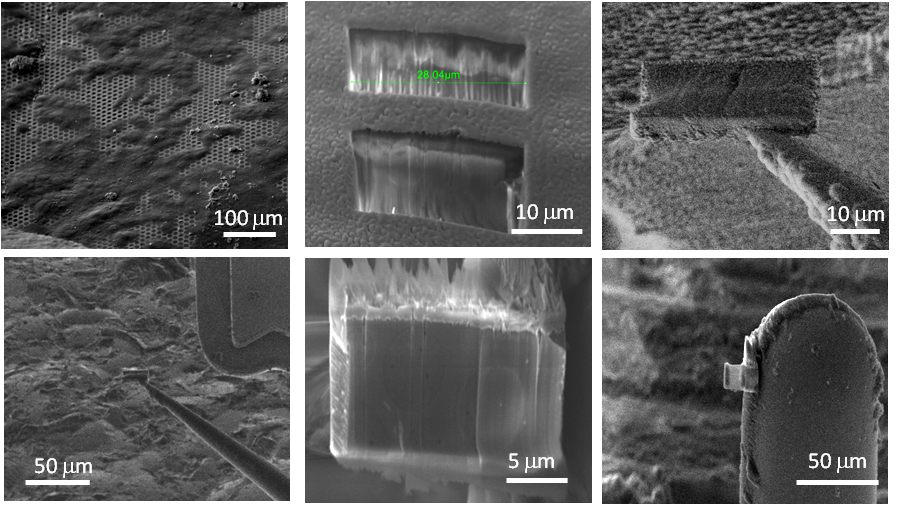
Process demonstrated on a yeast cell (Tesco value yeast) extracted as control sample to compare to literature results, which are well documented.
While the development of the cryo liftout solution was aimed at biological and battery technology, we have also seen it used for some unexpected applications. These include a TEM sample of toothpaste to look at the distribution of nano particles and a cryo tomography sample of ice-cream to investigate the relationship between the pore size on the fat molecules and the formation of ice crystals (this is what causes ice cream to become crunchy when refrozen).
To find out more about our cryo liftout solution contact one of our application specialists.
Contact Us