Correlative microscopy is a growing approach across all scientific fields. But it is often difficult to know where to start, particularly when considering the wide range of techniques that can be used. A recent chapter by Scher and Avinoam (2021) (in the Methods in Cell Biology book series), defines choosing the correct combination of imaging techniques to answer your research questions as the “microscopists dilemma”. Even identifying the amount of information you need to collate in order to design your experimental set-up can be a challenge. While this chapter is primarily aimed at biologists, the planning methodology it sets up can be applied to all areas of correlative research. So, where do you start?
The first thing you need to do is determine your research questions and identify your sample or samples. From there, the following framework can help you identify features that are most important to your investigation.
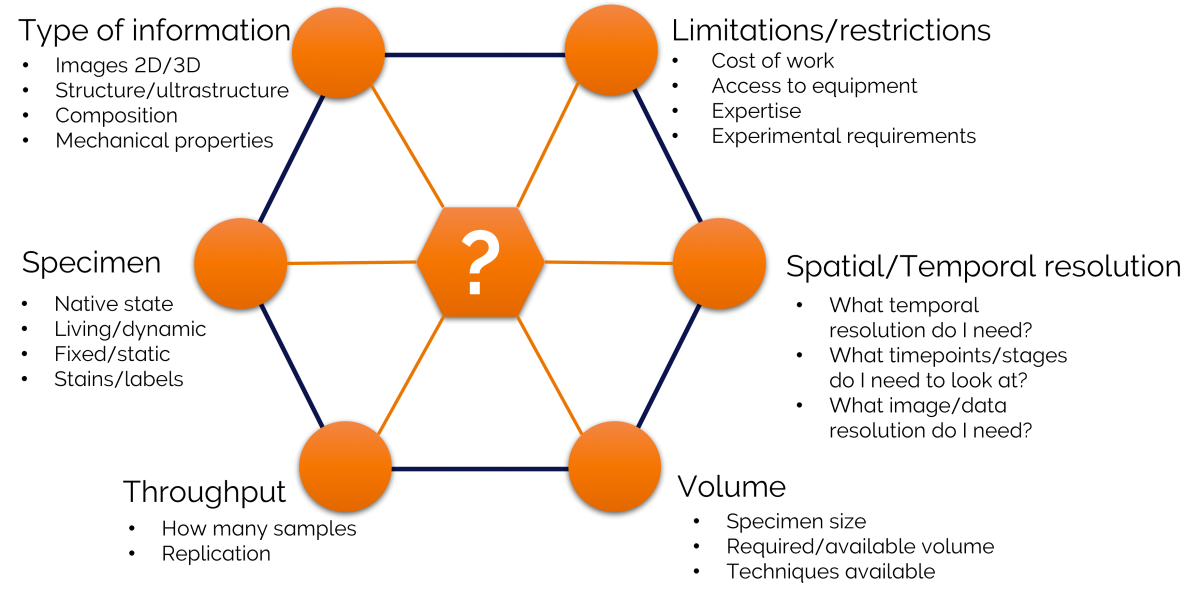
Figure 1 showing the types of questions that should be considered when determining the best imaging modalities for correlative microscopy techniques. Adapted from Scher and Avinoam (2021) Methods in Cell Biology volume 162: 1-11.
This type of framework can help to guide you towards identifying your imaging modalities and your workflow. All of the components in this framework interlink, with some affecting others to a greater or lesser extent depending on your field of research and your sample type. I have found that using this type of experimental planning is particularly important if you need to introduce additional steps, for example, if you need to modify your sample when moving between different types of microscopes.
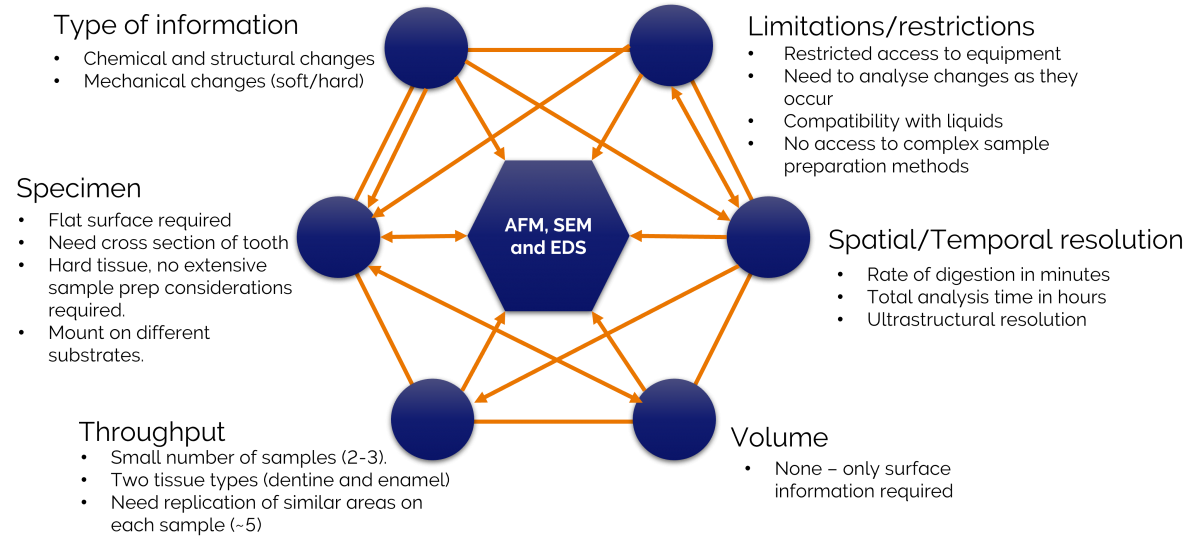
An example of using the framework is shown below and is from a preliminary investigation that I conducted with a colleague earlier this year. We wanted to quantify the changes that occurred in the chemistry and mechanical properties of dentine and enamel following acid digestion.
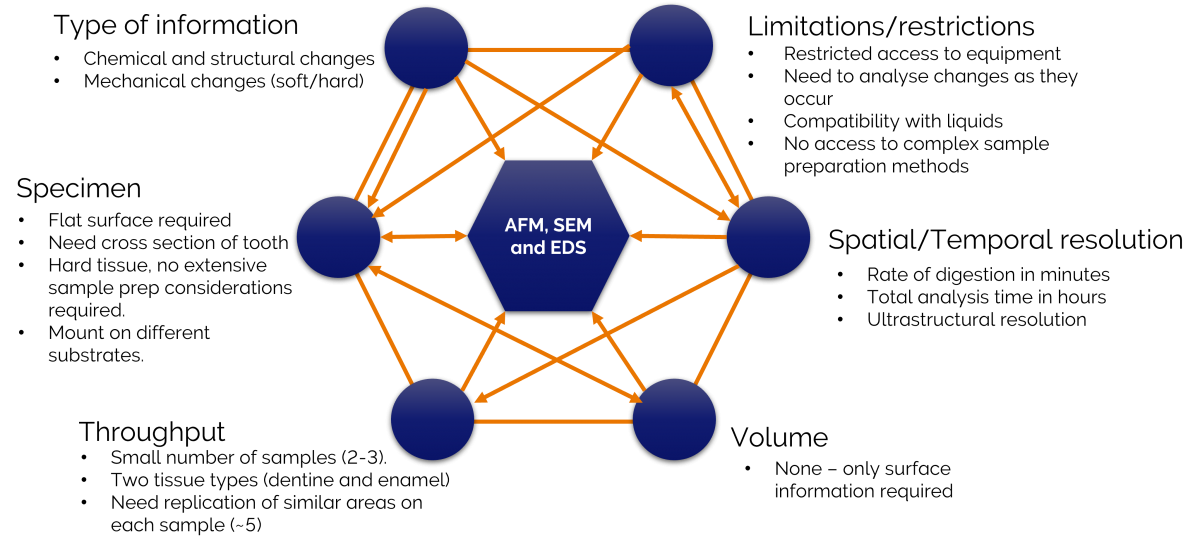
Figure 2 showing an application of the framework to study acid digestion in teeth.
The two parts of this analysis crucial to identifying our correlative imaging modalities were the type of information we were seeking and the limitations we had due to restricted access to equipment and lab facilities. Atomic force microscopy (AFM), scanning electron microscopy (SEM) and energy dispersive x-ray spectrometry (EDS) could provide the information we wanted. The identification of these imaging modalities, in-turn, led to us determining sample preparation methods, a realistic sample throughput, the type of resolution we might achieve and the overall workflow.
One of our main areas of interest was analysing changes while the sample was exposed to different acid solutions. This couldn’t be done in an electron microscope. The following workflow was thus established:
- Determine the composition and structure of the sample before digestion using SEM and EDS
- AFM analysis of topography and measurement of mechanical properties both before, during and after exposure to acid
- Repeat the SEM and EDS analysis for after-acid exposure measurements
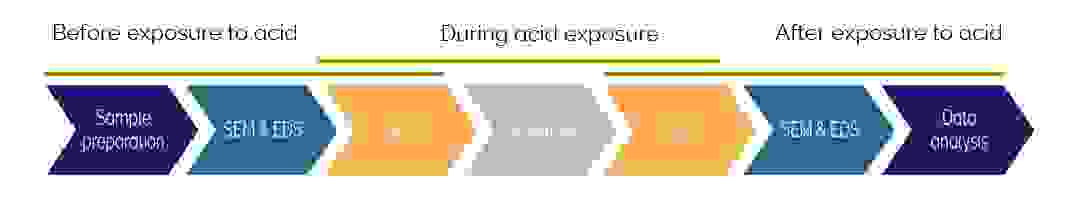
Figure 3 showing the imaging workflow for studying acid digestion of teeth.
Our workflow required the sample to be mounted onto different substrates between each imaging modality. We also needed to ensure that we didn’t use a conductive coating for the SEM analysis, as this would potentially interfere with AFM measurements. Planning the experiment first made a significant difference when performing our experiment and making sure these steps were accounted for in the first instance, reducing the amount of repetition and equipment time required. It was also important as it involved moving between not only microscopes but locations and users as well, which is often the case with correlative research.
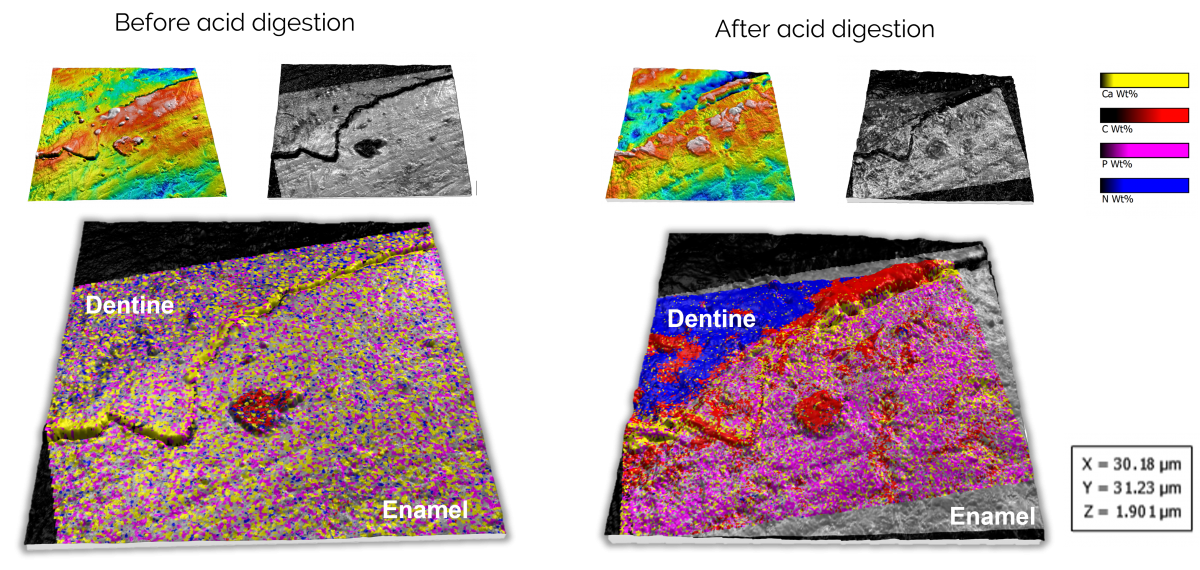
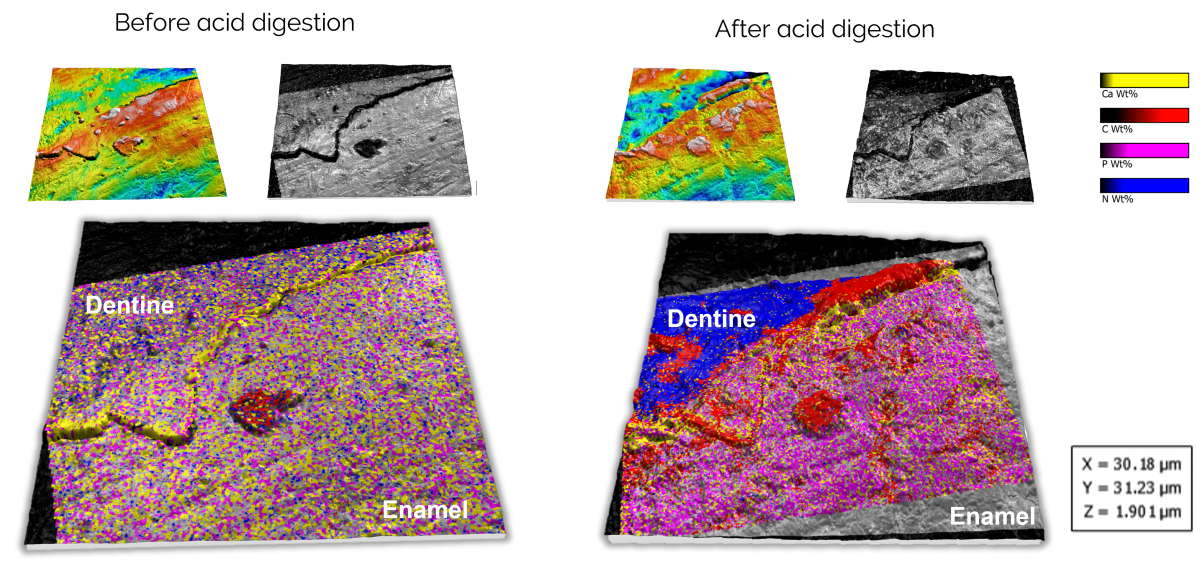
Figure 4. Correlated data on the effects of acid digestion on dentine and enamel. The alignment and visualisation were performed using Relate.
All of the data acquired in this study was correlated and analysed using our Relate software. If you are interested in finding out more about Relate and how it can help with analysing your correlative data, or to discuss your correlative project and how we can help, please get in touch with us on our Relate webpage or via the form below.
For more information on our methodology, results from this experiment and a step by step demonstration of our set-up, please take a look at our correlative microscopy tutorial, or check out my blog on techniques to find the same region of your sample on different microscopes.