Improvements in imaging and computing technology have resulted in the development of several electron microscopy (EM) techniques that enable high resolution 3D analysis of biological samples. The imaging revolution currently afoot is designated volume electron microscopy or volume EM (vEM) and has been in the spotlight as one of the technologies to watch in 2023.
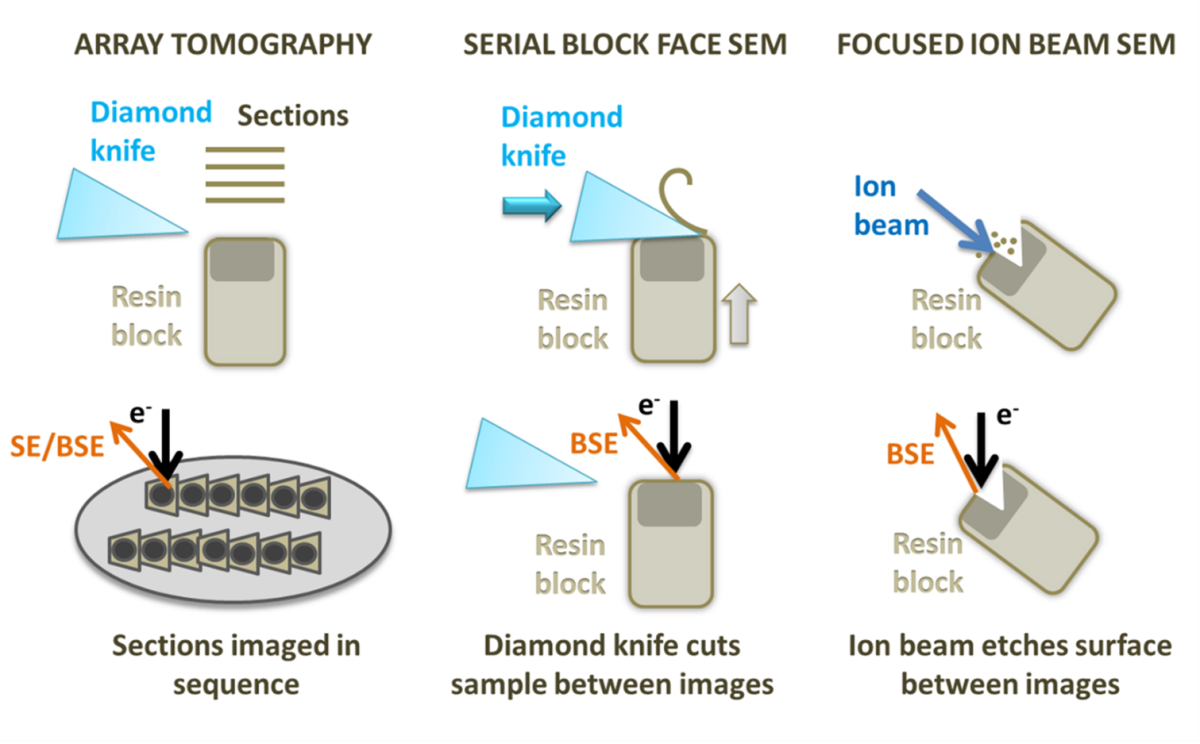
There is a variety of both scanning electron microscopy (SEM) and transmission electron microscopy (TEM) workflows for vEM from which I would like to highlight the SEM based techniques: Focused Ion Beam SEM (FIB-SEM), Serial Block Face SEM (SBF-SEM) and Array Tomography.
(Serial Section TEM tomography has a special place in my heart, I would need a blog post just for that one).
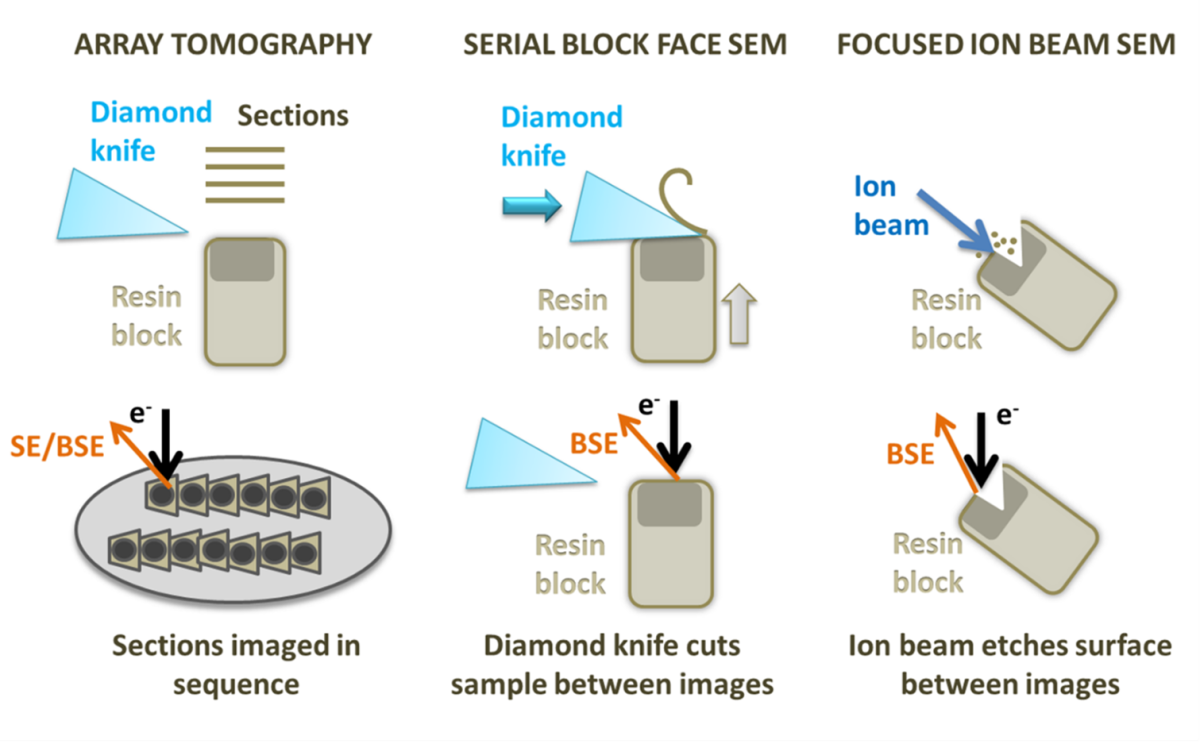
Diagram showing array tomography, serial block face SEM and focused ion beam SEM.
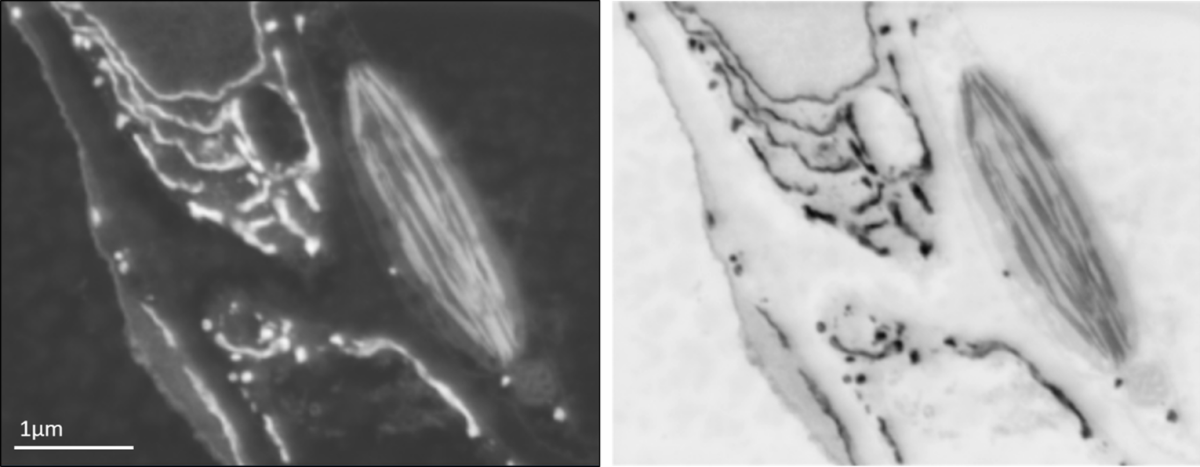
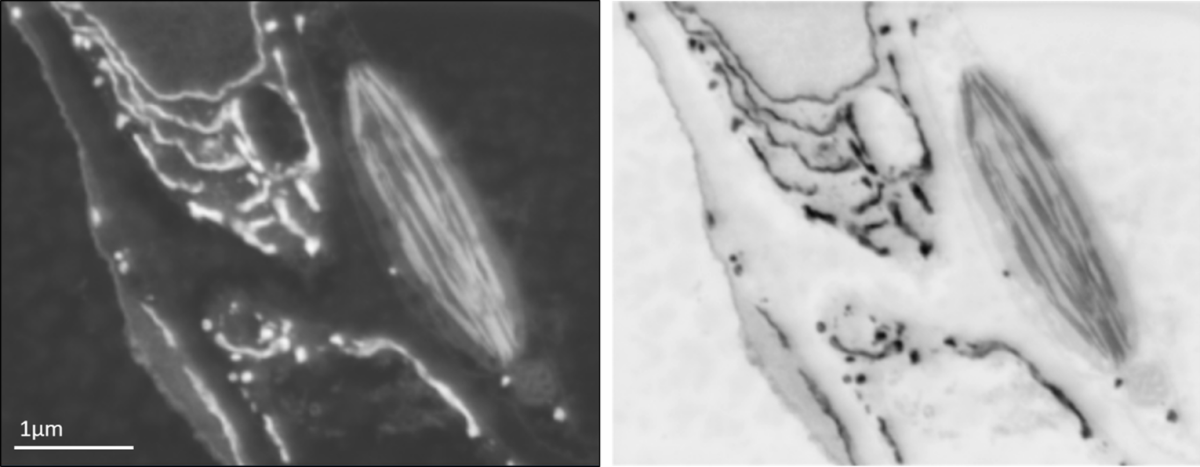
Backscattered electron SEM image of leaf tissue showing endoplasmic reticulum and a chloroplast (left) and the same image inverted for an image that appears similar to the type of data obtained using a TEM (right).
FIB-SEM
FIB-SEM utilises an ion beam to ablate the sample, creating a trench with a flat surface on one side that can be used for imaging with an electron beam. Once an image of the surface is collected, the ion beam is used to mill away a thin layer (3 nm up to tens of nm depending on the z resolution you need to achieve) from the imaging region. A second image is captured, and the process is repeated, building up a stack of images. FIB-SEM is also able to mill the sample to create a thin lamella that can be used as a TEM section, which is particularly useful for cryo applications (read the expert advice from Kim Larsen for selecting nanomanipulators and sample lift-out). FIB-SEM provides the best resolution in Z of all the 3D SEM techniques and can be used on frozen-hydrated samples and resin embedded room temperature specimens. The 3D volumes obtained with FIB-SEM can be acquired with isotropic resolution, which means that you can measure, render, and visualise your structures with the same precision in any direction. However, it also has the smallest field of view and lowest volume capacity of these methods and has the additional disadvantage of destroying the region of the sample being imaged. Development of workflows using plasma FIBs is trying to tackle larger volumes and produce flatter cryo-EM surfaces.
SBF-SEM
SBF-SEM uses a diamond knife mounted onto a microtome within the SEM specimen chamber to slice the surface of the specimen. The cut face of the sample is imaged before the knife is used to trim another section away. Slices as thin as 15 nm can be removed from very stable samples, but more often slices range from 20 to 100 nm. This means that the x and y resolution is usually better than the z resolution. On some samples and microscopes, it is possible to recover some extra z resolution using variable voltages for the electron beam. As with FIB-SEM, SBF-SEM is a destructive method, and it is not possible to revisit sections later. SBF-SEM is also restricted to room temperature samples with a lot of contrast. SBF-SEM can image a relatively large volume of tissue (hundreds of microns) and a large surface area, avoiding section specific artefacts, such as compression and wrinkling. The resin block can be prone to beam damage and charging, requiring measures, such as using a gas injection system or variable pressure in the microscope or very low accelerating voltages, which reduces contrast. SBF-SEM is the fastest method for set up and data collection over a specified volume of all the techniques.
Array tomography
Array tomography involves producing serial sections on an ultramicrotome and mounting them onto a stub or wafer before inserting them into the microscope. Each section is imaged in sequence, and the same region can be revisited and imaged multiple times at different magnifications and resolution. Stains and immunolabels can be applied to the sample after embedding, and after imaging once regions of interest have been located, something that cannot currently be done with SBF-SEM. Array tomography does not have a limit on the volume of tissue that can be imaged and is the most flexible system. However, the z resolution is restricted by the thickness of the sections (30 nm at best) and it can be prone to wrinkles, compression and other section-specific artefacts.
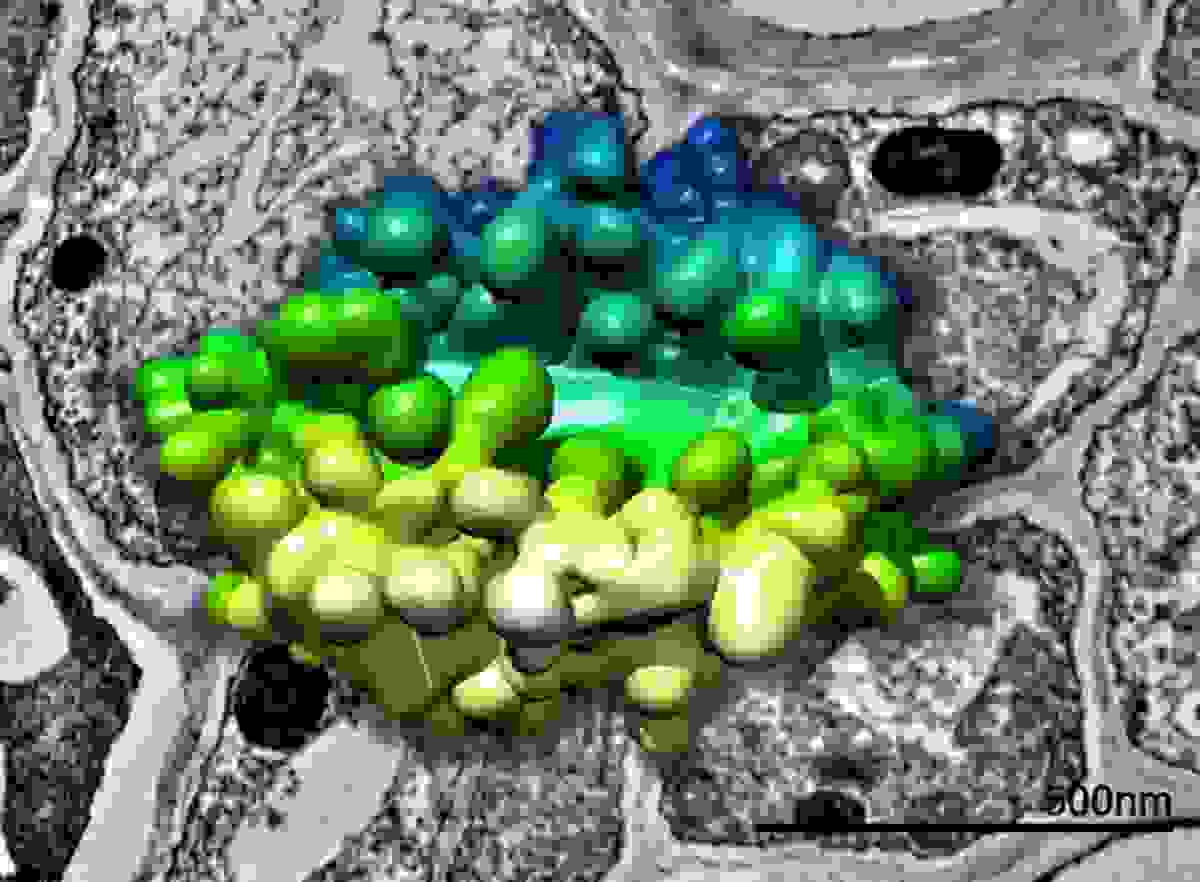
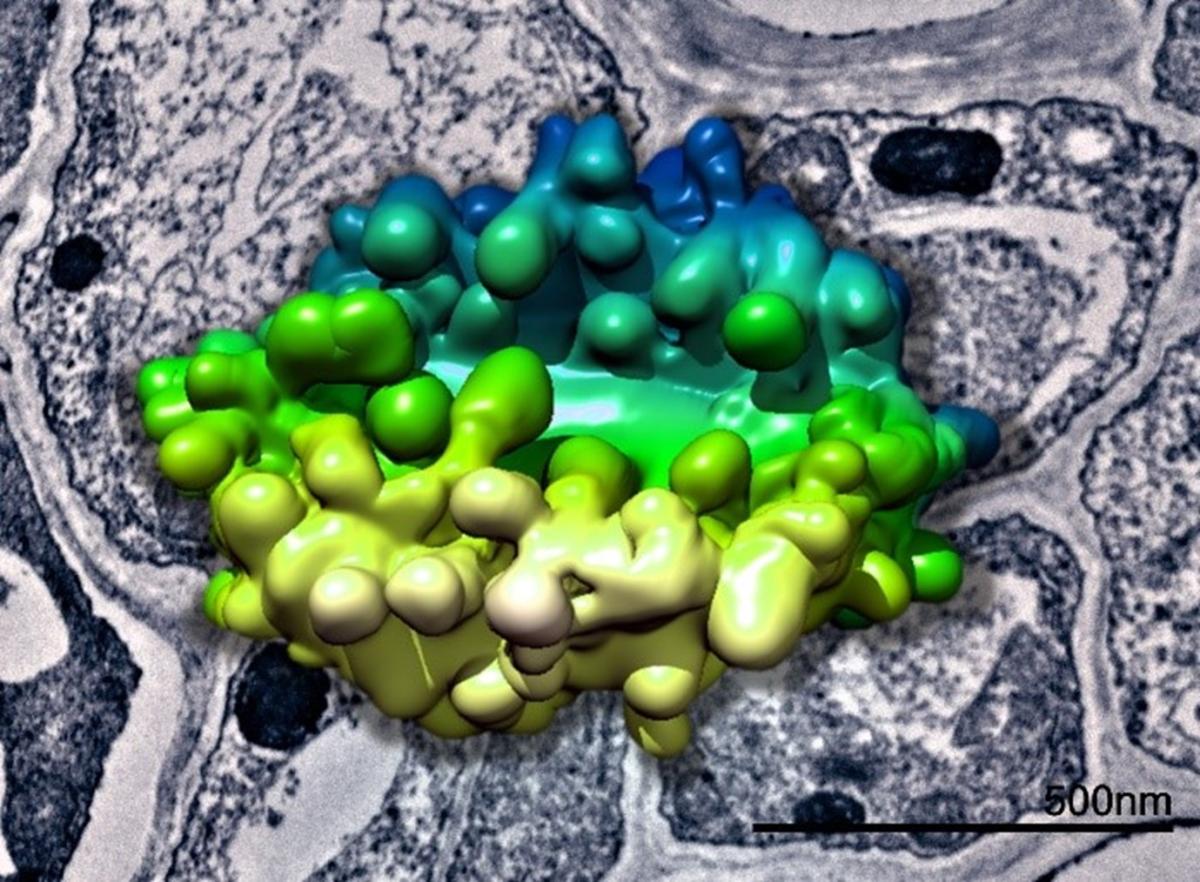
3D model of a plant Golgi body generated from SBF-SEM data superimposed above a TEM image of plant tissue. Data collected in collaboration with Chris Hawes.
AZtec solutions for your vEM imaging workflows
All the 3D SEM techniques produce large volumes of data that require significant imaging, processing, and analysis time.
If you have tried any of the workflows above, you have probably faced challenges navigating your sample and finding your imaging regions (often correlating multimodal data), especially with specimens that can range from micron to several millimetres large. And with such long imaging running times, accessing your SEM remotely has never been more important (tracking the imaging, changing conditions on-the-fly, setting up overnight runs).
With AZtec software functionalities, all those challenges can be tackled: remote access for imaging (check out this blog post by Anthony Hyde), easy navigation with stage control using the Enhanced Sample Navigation functionality, Large Area Mapping for automated acquisition of large surfaces (see this in action in this blog post by Lucia Spasevski, and collecting 3dimensional data with AZtec3d.
To learn more about how AZtec and EDS can be a part of your vEM workflows please visit our Bio-Imaging & Life Science application page.