Forensics
Obtaining reliable and reproducible forensic evidence is critical to ensure it can undergo scrutiny in a court of law. Composition information is required on a wide range of forensics samples, from gunshot residue, to soil and minerals. This information can be obtained non-destructively using a SEM and X-ray microanalysis techniques (e.g., EDS, WDS). When key elements are present in low concentrations and EDS cannot produce an accurate enough result, or there is uncertainty over the presence of an element, WDS can provide the detection levels (<100ppm for many elements) required.
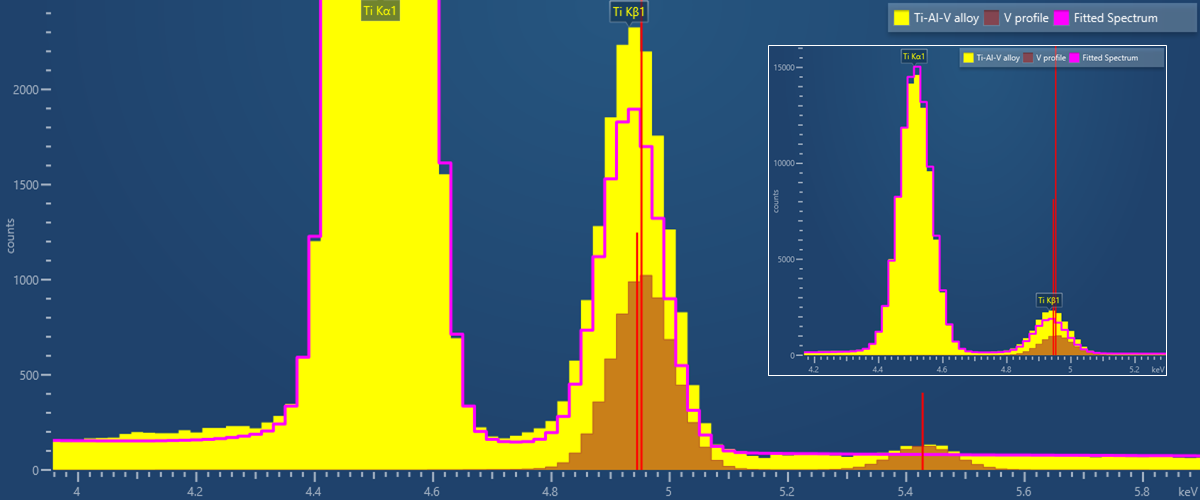
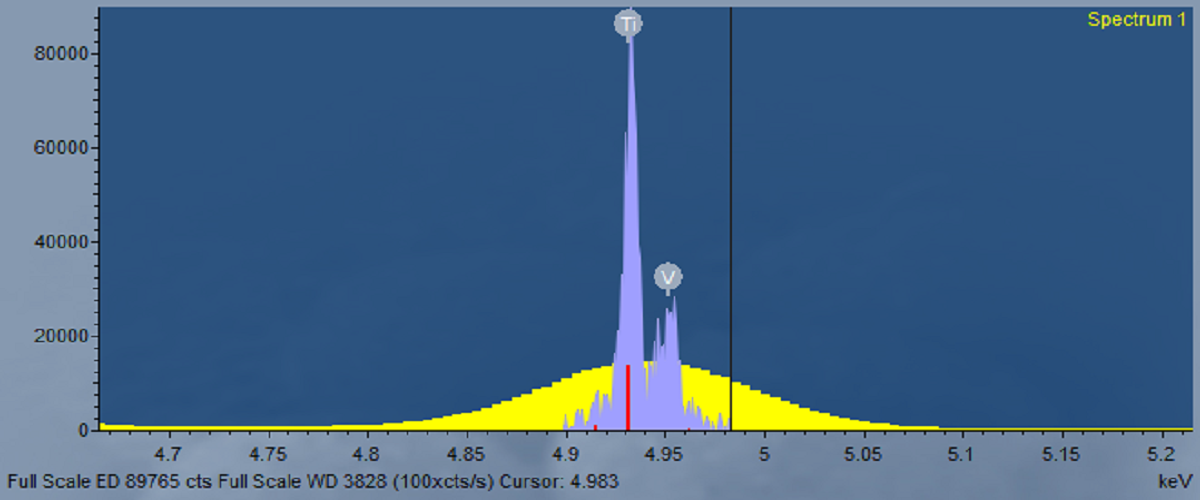
Here we present some data collected from glass of known composition using AZtecWave. These measurements were made using an Ultim Max 100 mm2 EDS detector, combined with a Max+ interface, and a WaveWDS spectrometer. The combined EDS-WDS analysis was carried out using an accelerating voltage of 20kV and a beam current of 82 nA. The minor/trace element (Mg, S, Ti, Fe, As and Ba) compositions were measured using WDS, and the major elements were determined by EDS. Known pure elements and simple compounds were measured as WDS standards prior to the quantitative analysis of the glass sample. The in-built, factory standards, provided in the AZtec software, were used for the EDS. As demonstrated in the below table, a good match is observed between the measured and published compositions.
WDS Analytical Conditions and Results
| Element |
O |
Na |
Mg |
Al |
Si |
S |
K |
Ca |
Ti |
Fe |
As |
Ba |
Total |
| Glass reference (wt. %) |
46.1 |
9.44 |
0.16 |
1.46 |
33.22 |
0.05 |
1.67 |
7.64 |
0.008 |
0.03 |
0.02 |
0.11 |
99.9 |
| Technique |
Cal. |
EDS |
WDS |
EDS |
EDS |
WDS |
EDS |
EDS |
WDS |
WDS |
WDS |
WDS |
- |
| WDS peak live time (s) |
|
|
10 |
|
|
30 |
|
|
150 |
150 |
150 |
50 |
|
| Average (wt. %) |
46.02 |
8.86 |
0.154 |
1.51 |
33.46 |
0.033 |
1.58 |
7.43 |
0.008 |
0.028 |
0.020 |
0.085 |
99.2 |
| 1σ |
0.08 |
0.09 |
0.004 |
0.03 |
0.06 |
0.002 |
0.01 |
0.07 |
0.001 |
0.001 |
0.001 |
0.011 |
0.2 |
*Table: Quantitative, combined EDS-WDS results obtained from a glass standard of known composition using AZtecWave. Oxygen has been calculated by stoichiometry.